In today’s competitive landscape, expanding to global markets requires a strategic approach to meet various countries’ regulatory standards. C-PRAV Certification Services Pty Ltd specializes in providing end-to-end Global Market Access (GMA) services, enabling our clients to achieve compliance across multiple regions, including the European Union (EU), Asia, Africa, Middle East countries and more.
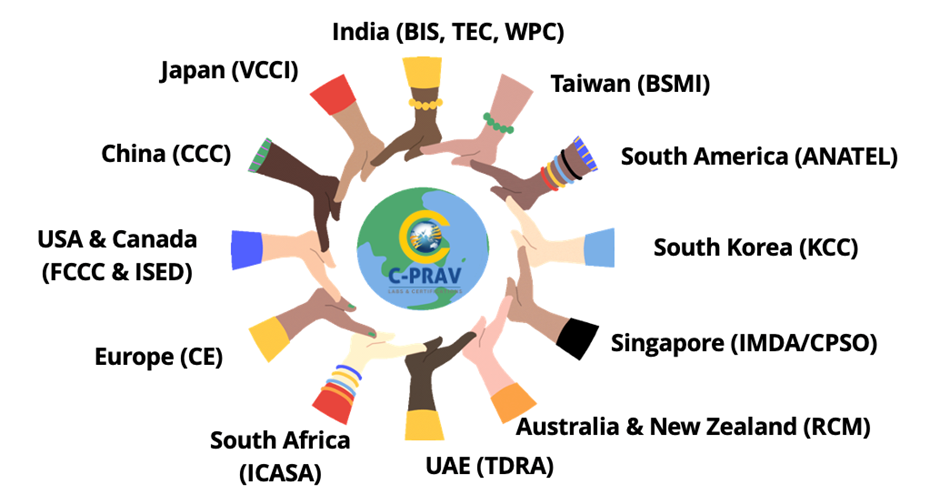
C-PRAV has completed approvals in more than 100 countries – managing regulatory complexities, ensuring smooth market entry, and maximizing your product’s reach worldwide.

Scope of Services
We provide a comprehensive suite of services for Global Market Access, customized to suit the specific requirements of your industry and target markets.
Regulatory Consultation and Strategy Development: Understanding the unique requirements of each market and developing a tailored compliance strategy.
Accredited Test Reports: Coordinating with accredited labs to obtain recognized test reports necessary for certifications, including radio and wireless compliance.
Product Certification and Documentation: Assisting with obtaining certifications such as CE (EU), FCC (USA), IC (Canada), ICSA (South Africa), SDPPI (Indonesia), KCC (South Korea) and other market-specific approvals.
Labelling and Documentation Requirements: Ensuring your product meets all labelling and documentation requirements specific to each region.
Technical File Compilation: Organizing and maintaining comprehensive technical files as per regulatory requirements.
Ongoing Compliance Management: Monitoring regulatory changes and providing guidance to ensure continued compliance after market entry.
To streamline the certification process and achieve faster market access, C-PRAV follows the phased approach:
Identifying applicable standards and requirements for each target market. Developing a roadmap for product compliance, including necessary testing, documentation, and timelines.
Testing at C-PRAV Labs or collaborating with in-country labs to conduct all required testing. Managing end-to-end right from Samples to Certificate. Compiling test reports and technical files to meet regulatory standards.
Submitting all documentation to relevant authorities or certification bodies. Coordinating closely with regulatory agencies to address queries or feedback.
Providing ongoing support to monitor regulatory changes and updates. Assisting with periodic renewals or recertifications as required.


C-PRAV Certification Services has strategically located offices and an extensive network of reliable partners across the globe with whom we work closely to complete the approvals in those specific countries. We are committed to delivering seamless Global Market Access services – leveraging our extensive industry experience, global network of accredited testing partners, and in-depth knowledge of international regulatory landscapes to offer our clients:
Efficiency: Time-bound project management to meet critical timelines.
Expertise: IT and technical background to handle complex, high-tech products.
Reliability: Ongoing support post-market entry to ensure sustained compliance.
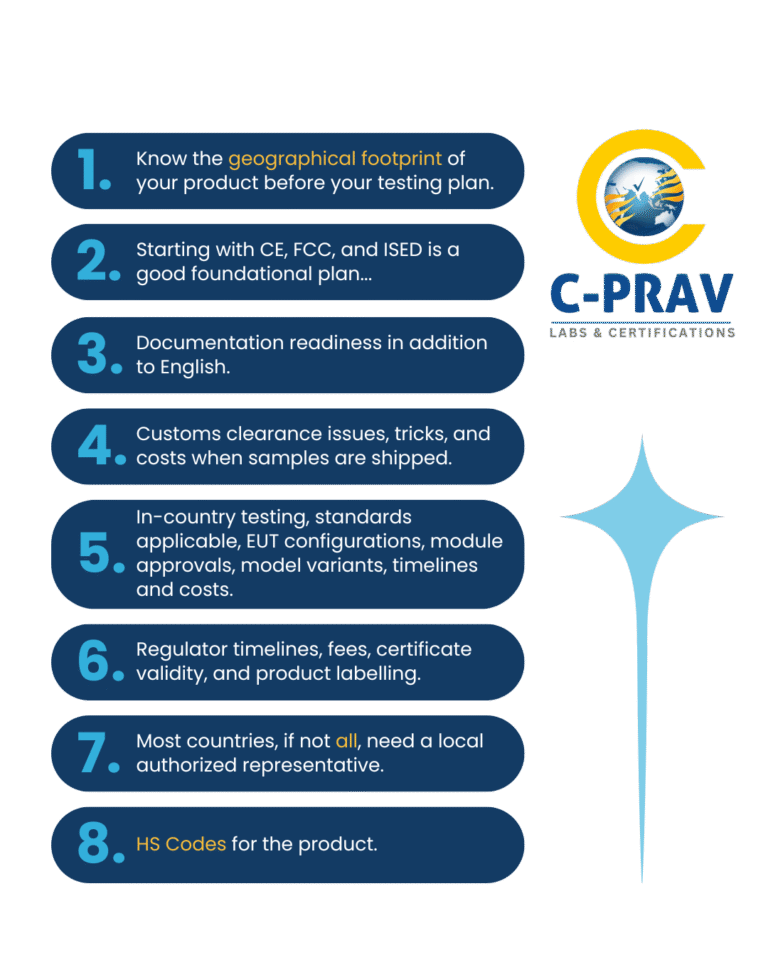
The Way Global Approvals Work
The expert help and support you need – from a knowledgeable and reliable partner.